The Ministry of Health, Labour and Welfare (MHLW) in Japan announced “DASH for SaMD” in 2020, a practical application promotion package strategy for medical device software (SaMD, Software as a Medical Device). While the environment for the practical application of SaMD has been improving mainly in Europe and the U.S., there had been concerns such as a “SaMD lag” (delay in practical application) in Japan. The objective of this package strategy is to promote the early commercialization of innovative cutting-edge SaMD.
|
DASH for SaMD DX (Digital Transformation) Action Strategies in |
✓ Early identification of budding seeds of innovative SaMDs and presentation of the concept of review
✓ Establishment of a centralized consultation office and a review system and structure based on the characteristics of each SaMD.
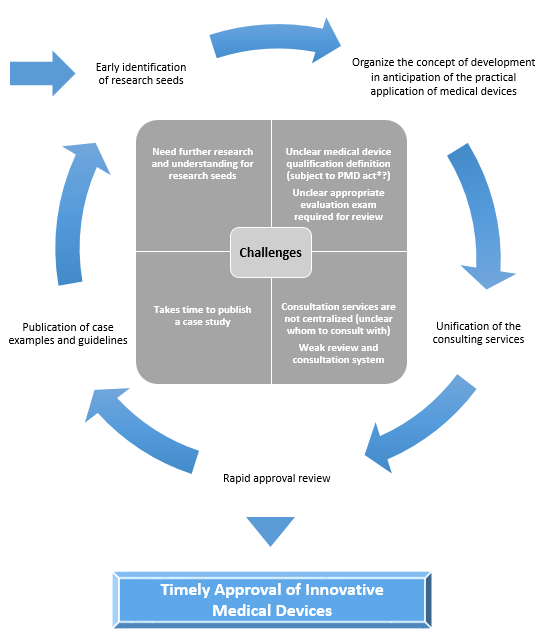
Challenges and Policies for the Promotion of Practical Use
*PMD act: Japan’s Pharmaceuticals and Medical Devices Act
DASH for SaMD, SaMD Practical Application Promotion Package Strategy
|
1. Early identification of seeds and announcement of reviewing approaches |
|
・Early identification of research seeds |
|
2. Unification of consultation service contacts |
|
・Centralize consultation services |
|
3. Review system based on characteristics of SaMD |
|
・Carry out efficient review based on characteristics of SaMD |
|
4. Enhance structure for early realization |
|
・Establish new section specialized in reviewing SaMD in PMDA and enhance structure of MHLW |
Micron offers a full range of services for obtaining certification/approval of SaMD, including consultation, study planning and operation, and handling of PMDA approval applications.
Please feel free to contact us for more information.
Recommendations
Articles, Services and Solutions
SaMD Approval Support Achievements
References