Our Systems
Micron offers two powerful image management and real-time visualization tools – IRUMneo and i-Boarding.
Our systems have been used in global clinical trials for more than 10 years. Sponsors and CRAs have access to both systems. In addition, Micron can customize reports needed from these systems for your unique needs.
IRUMneo and i-Boarding
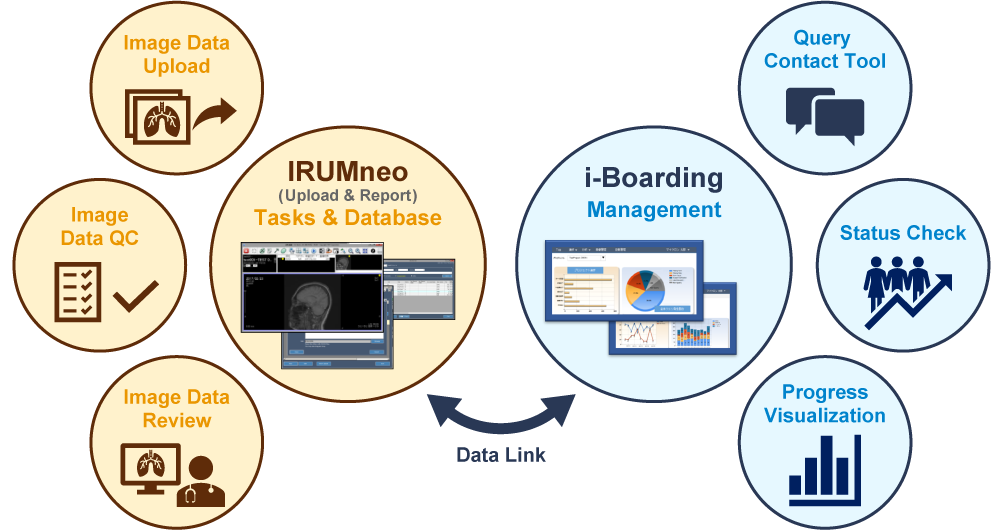
IRUMneo is a proprietary system that implements the imaging study workflow of data uploads, data QC, and interpretation in one system which is available 24 hours a day, from anywhere in the world.
i-Boarding is an image progress management system. It is linked with IRUMneo to provide query communication, progress checks, and analysis functions.
IRUMneo
Image Data Upload

The data uploading system within IRUMneo supports various types of images such as DICOM images, videos, or still images. The uploading process is simple and provides a very good uploading speed. Feedback from our customers is positive regarding our upload ease of use.
Image Data QC
Images can be viewed immediately in IRUMneo after uploading and the quality can be quickly checked. During uploading, IRUMneo automatically checks DICOM tags for common concerns such as patient names and other personal information. Access to IRUMneo for sponsors and CRAs can also be granted.
Image Data Review

IRUMneo can be customized according to specific clinical trial needs such as eligibility, safety and specific response criterion. Examples may include RECIST 1.1, irRECIST, or the Lugano Classification.
The reading system can be adapted to various reading styles per the central read requirement: a single read by one independent reader, an adjudicator who checks the results of two independent readers, or multiple independent readers.
i-Boarding
Query Contact Tool

When an inquiry arises for received image data, it is possible to communicate the details using an i-Boarding chat function. Each query registered in the system is automatically sent to the other party by email, enabling secure and timely responses.
Queries are managed and tracked automatically, including flagging overdue queries. Our team reviews queries to ensure they are responded to in a timely and efficient manner, including making a phone call.
Status Check
In real time, i-Boarding automatically displays and analyzes data metrics including data image receipt status, image QC results, and query status. Other viewable real time metrics include: the time for each site to submit data, site query classification, and the percentage of outstanding queries. Customizable reports can also be created.


The data uploading system within IRUMneo supports various types of images such as DICOM images, videos, or still images. The uploading process is simple and provides a very good uploading speed. Feedback from our customers is positive regarding our upload ease of use.
Images can be viewed immediately in IRUMneo after uploading and the quality can be quickly checked. During uploading, IRUMneo automatically checks DICOM tags for common concerns such as patient names and other personal information. Access to IRUMneo for sponsors and CRAs can also be granted.

Image Data Review

IRUMneo can be customized according to specific clinical trial needs such as eligibility, safety and specific response criterion. Examples may include RECIST 1.1, irRECIST, or the Lugano Classification.
The reading system can be adapted to various reading styles per the central read requirement: a single read by one independent reader, an adjudicator who checks the results of two independent readers, or multiple independent readers.
i-Boarding
Query Contact Tool

When an inquiry arises for received image data, it is possible to communicate the details using an i-Boarding chat function. Each query registered in the system is automatically sent to the other party by email, enabling secure and timely responses.
Queries are managed and tracked automatically, including flagging overdue queries. Our team reviews queries to ensure they are responded to in a timely and efficient manner, including making a phone call.
Status Check
In real time, i-Boarding automatically displays and analyzes data metrics including data image receipt status, image QC results, and query status. Other viewable real time metrics include: the time for each site to submit data, site query classification, and the percentage of outstanding queries. Customizable reports can also be created.


IRUMneo can be customized according to specific clinical trial needs such as eligibility, safety and specific response criterion. Examples may include RECIST 1.1, irRECIST, or the Lugano Classification.
The reading system can be adapted to various reading styles per the central read requirement: a single read by one independent reader, an adjudicator who checks the results of two independent readers, or multiple independent readers.

When an inquiry arises for received image data, it is possible to communicate the details using an i-Boarding chat function. Each query registered in the system is automatically sent to the other party by email, enabling secure and timely responses.
Queries are managed and tracked automatically, including flagging overdue queries. Our team reviews queries to ensure they are responded to in a timely and efficient manner, including making a phone call.
Status Check
In real time, i-Boarding automatically displays and analyzes data metrics including data image receipt status, image QC results, and query status. Other viewable real time metrics include: the time for each site to submit data, site query classification, and the percentage of outstanding queries. Customizable reports can also be created.

In real time, i-Boarding automatically displays and analyzes data metrics including data image receipt status, image QC results, and query status. Other viewable real time metrics include: the time for each site to submit data, site query classification, and the percentage of outstanding queries. Customizable reports can also be created.

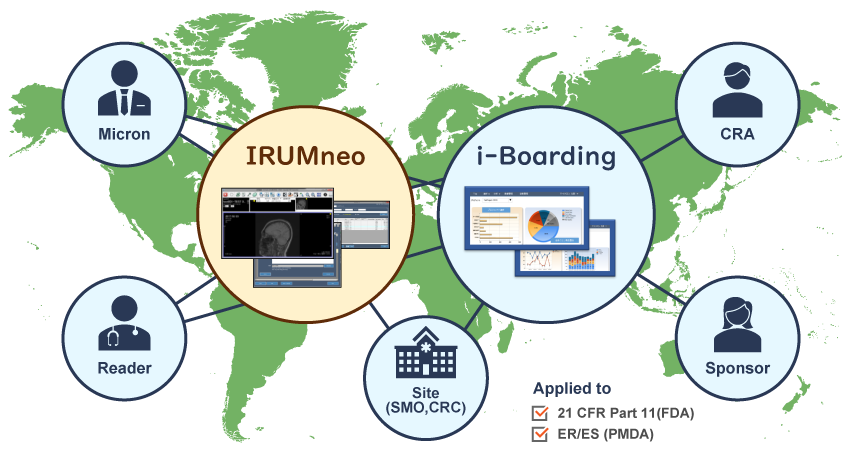
See the IRUMneo and i-Boarding demonstration video here
IRUM and i-Boarding are registered trade marks of Micron, Inc.
